|
Publication 77: Optimization of Gas–liquid Photochemical Reactions using an Automated Flow Platform

Stevens, Dorbec et al. have developed an automated continuous flow-through platform based around a Borealis Flow photoreactor capable of optimising photocatalytic gas-liquid flow processes.
A Bayesian optimisation approach was used to enable a 14-fold increase in productivity for the oxidation of tetralin.
Studies on a further 9 substrates showed the platform to be a useful tool for reaction optimisation and process intensification.
|
|
|
|
Publication 76: Improved Photocatalysed Csp2-Csp3 Cross-coupling of Alkylarenes to Aldehydes under Continuous Flow Conditions

Executing the photoredox-catalysed cross-coupling of alkylarenes with aldehydes under continuous flow conditions was demonstrated to improve yields, reduce reaction times and improve throughput.
It was possible to recycle the iridium photocatalyst several times without a significant reduction in efficacy.
A throughput in excess of 10g in an 8 hour period was achieved using the Uniqsis PhotoSyn high-power 365nm light source.
O. M. Griffths, H. A. Esteves, Y. Chen, K. Sowa, O. S. May, P. Morse, D. C. Blakemore, and S. V. Ley, J. Org. Chem. 2021, 86, 13559-13571
|
|
|
|
Publication 75: Synthesis of Spirocyclic Tetrahydronapthyridines from Primary Alkylamines
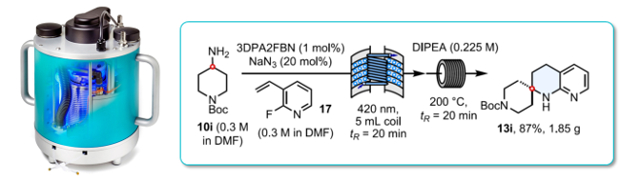
Photoredox-catalysed hydroaminoalkylation of halogenated vinyl pyridines followed by an intramolecular SNAr cyclisation affords a concise synthesis of tetrahydronapthyridines in high yields under continuous flow-through conditions.
Sterically constrained tetrahydronapthyridines are a valuable scaffold in drug discovery.
The photo-catalysed HAA reaction was found to work most efficiently at 420nm using a Uniqsis PhotoSyn photoflow reactor.
|
|
|
|
Publication 74: Flow Synthesis of Non-steroidal, Anti-inflammatory COX-2 Inhibitor Celecoxib
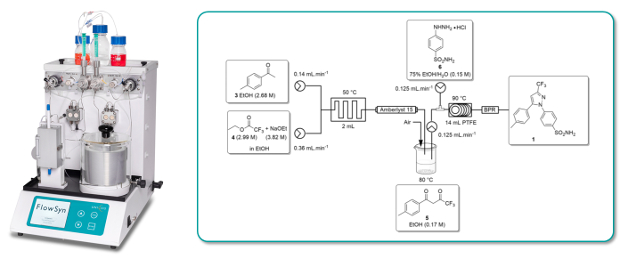
A high yielding (90%) continuous flow-through synthesis of the non-steroidal, anti-inflammatory COX-2 inhibitor Celecoxib has been developed using a FlowSyn continuous flow reactor system.
The individual steps of the 2-stage process were first optimised independently in both batch and flow before being combined for the flow synthesis.
Whereas the corresponding batch process required 20h, the flow through synthesis was complete in a residence time of only 1 hour.
|
|
|
|
Publication 73: C-H Alkylation of Unmasked Primary Amines under Photocatalytic HAT Conditions
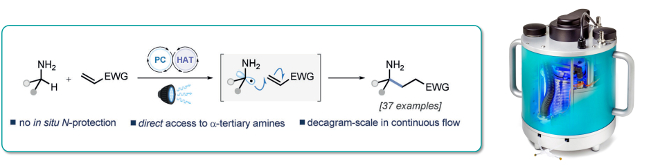
A photocatalytic protocol for the a-C-H alkylation of unprotected primary amines that is amenable to the direct synthesis of alpha-tertiary primary amines is reported.
The reaction has broad scope and affords good yields with a broad range of substrates.
The PhotoSyn was utilised to demonstrate that this process is readily scalable in continuous flow to provide access to decagram quantities of valuable gamma-lactams and azaspirocycles, for application in drug discovery.
A. S. H. Ryder, W. B. Cunningham, G. Ballantyne, T. Mules, A. G. Kinsella, J. Turner-Dore, C. M. Alder, L. J. Edwards, B. S. J. McKay, M. N. Grayson, A. J. Cresswell, Angew. Chem. Int. Ed. 2020, 59, 35, 14986-14991
|
|
|
|
Publication 72: A One-Pot Photochemical Method for the Generation of Functionalised Aminocyclopentanes
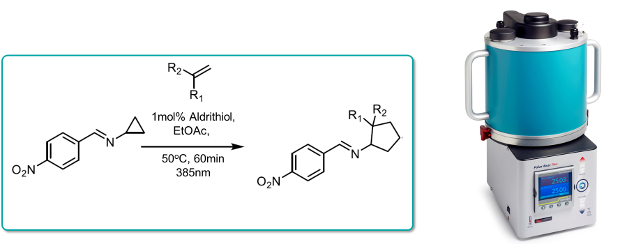
Iminocyclopropanes were converted into functionalised aminocyclopropanes in the presence of functionalised alkenes in a formal [3+2] cycloaddition. Formation of the requisite N-centred diyl radical was promoted under photochemical conditions by irradiation with violet light.
The reaction could be considerably accelerated under continuous flow-through conditions in the Uniqsis PhotoSyn at 385nm in the presence of Aldrithiol as a polymer inhibiting agent (4 - 90min) at 500C, thereby facilitating scale-up.
|
|
|
|
Publication 71: Use of Open-Source Software to Implement Closed-Loop Reaction Optimisation under Flow-Through Conditions
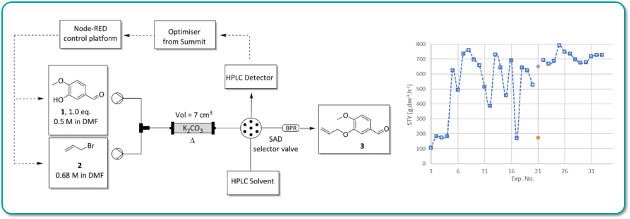
Researchers at the University of Pretoria (South Africa) have developed a generalplatform for performing closed loop reaction optimisation by integrating a Uniqsis FlowSyn Maxi continuous flow reactor with an analytical HPLC.
The FlowSyn Maxi was controlled over ethernet using an open-source Node-Red dashboard running on a Raspberry Pi linked to the single objective optimisation algorithm SUMMIT.
An autonomous optimisation of a representative allylation reaction in flow was performed over 33 iterations in a 12-hour period.
|
|
|
|
Publication 70: Gram Scale Synthesis of Azetidinium Salts in Flow
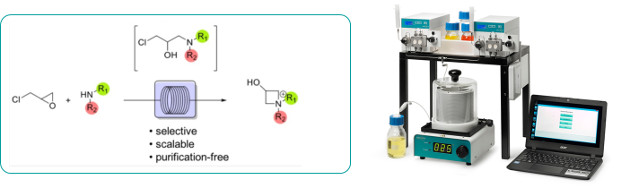
Vile and co-workers have reported a practical procedure for the on-demand preparation of synthetically useful azetidinium salts via aminolysis of epichlorohydrin under continuous flow conditions.
Using the Uniqsis FlowLab flow chemistry reactor system, the procedure is both simple and scalable affording high yields of products that can be isolated directly within 60 minutes at 80oC.
Typically higher yields are obtained in flow than in the corresponding batch process.
|
|
|
|
Publication 69: Photocatalytic Hydroaminoalkylation of Styrenes with Unprotected
Primary Alkylamines

Cresswell has reported a catalytic, organophotoredox procedure for the intermolecular hydroaminoalkylation (HAA) of styrenes to afford pharmacologically relevant gama-arylamines that does not require protection of the amine.
The reaction tolerates a broad range of substrate functionalities to afford in good yields.
Moreover, the reaction can be combined with a subsequent intramolecular N-arylation to produce tetrahydroquinolines.
H. E. Askey, J. D. Grayson, J. D. Tibbetts, J. C. Turner-Dore, J. M. Holmes, G. Kociok-Kohn, G. L. Wrigley, and A. J. Cresswell, J. Am. Chem. Soc., 2021, DOI: 10.1021/jacs.1c07401
|
|
|
|
Publication 68: Multistep Continuous Flow Synthesis of Visible-Light-Mediated
Photochemistry with a High-Temperature Cascade Reaction
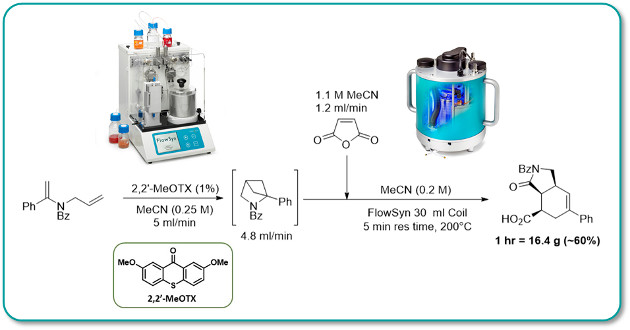
A highly efficient visible-light-mediated photochemical [2 + 2] cycloaddition is coupled with an atom economic thermal cascade reaction in a continuous flow process.
By moving the photochemistry from ultraviolet (UV) to visible, it is made more energy-efficient and can be conducted with readily available equipment. The application of high-temperature flow chemistry to the thermal cascade step allows for safe and reliable scale-up with short reaction times.
This sequential photo/thermal transformation was performed with a throughput of 20g/hour using commercially available instrumentation.
The synthetic utility of flow chemistry is further showcased by the isolation of a reactive intermediate at quantities not possible under batch conditions.
|
|
|
|
Publication 67: Fast Screening and Optimisation of a RAFT Polymerisation in Micro-Scale Reactors
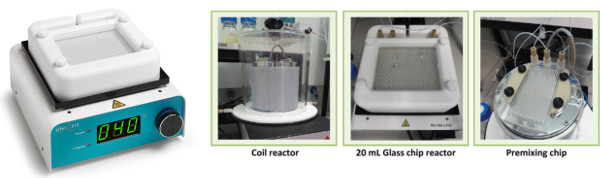
Two glass chip reactors and a coil reactor are used in different combinations to investigate the RAFT polymerisation of MMA in a micro-scale continuous flow reactor system.
Optimisation was facilitated by the use of in-line NMR analysis in combination with kinetic and multiphysical modeling to find the optimal process parameters (temperature and residence time) as well as the best composition of the reaction mixture in order to optimize the conversion and molecular characteristics of the synthesized polymer.
|
|
|
|
Publication 66: Selective Zincation of 1,2-Dicyanobenzene and Related Benzonitriles Using In Situ Trapping Metalations Under Continuous Flow Conditions
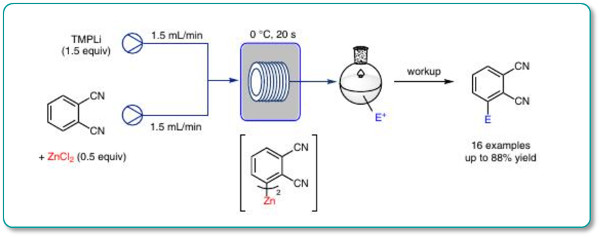
A mild and convenient metalation procedure for the functionalization of 1,2-dicyanobenzene and related polyfunctionalized benzonitriles using the Uniqsis FlowSyn is reported.
The addition of TMPLi to a mixture of an aromatic substrate with a metallic salt such as ZnCl2 under appropriate conditions (0 °C, 20 s) leads to fast in situ lithiation of the arene followed by transmetalation with ZnCl2 to afford the corresponding functionalized arylzinc compounds.
Trapping with various electrophiles afforded the adducts in high yields.
The reaction scope of these in situ trapping metalations in flow is broader and needs less equivalents of the base and the metal salt than the corresponding batch procedure.
|
|
|
|
Publication 65: A Photoredox Coupling of Benzylboronic Esters and
Carbonyl Compounds in Flow
.gif)
Efficient cross-coupling of benzylic organoboron reagents with carbonyl compounds under flow-through photoredox conditions has been demonstrated and shown to be exhibit wide functional group tolerance.
Preliminary results also show the reactions could be scaled using more powerful flow photoreactors such as the PhotoSyn coupled to computer-controlled flow chemistry devices.
A Uniqsis Flow-UV UV/Vis spectrometer was used in-line to monitor steady state.
|
|
|
|
Publication 64: Preparation of Silver Nanowires under Segmented Flow-through Conditions

The effect of chain length of PVP on the structural and morphological properties of AgNWs produced using a segmented flow reaction constructed using a Uniqsis BPM and a HotCoil was investigated.
A higher PVP chain length led to a smaller diameter and longer length of the AgNWs. The electric properties of AgNWs exhibited high stability toward bending up to 200 cycles.
Long and thin AgNWs can be produced by a flow synthesis process which potentially can be used in flexible TCE applications.
K. S. Lau, S. X. Chin, S. T. Tan, F. S. Lim, W. S. Chang, C. C. Yap, M. H. H. Jumali, S. Zakaria, S. W. Chook, C. H. Chia, Journal of Alloys and Compounds 2019, 803, 171
|
|
|
|
Publication 63: Application of a Preformed Pd-BIDIME Precatalyst to Suzuki-
Miyaura Cross-Coupling Reaction in Flow
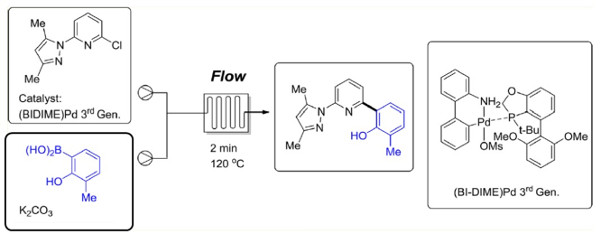
The application of a pre-formed 3rd generation Buchwald palladacycle containing a BIDIME ligand under was used to prepare a key biaryl intermediate in high yield and purity.
Under continuous flow conditions low conversions associated with catalyst deactivation under batch conditions were avoided and it was possible to utilise a short residence time under superheated conditions.
J. D. Sieber, F. Buono , A. Brusoe, J.-N. Desrosiers, N. Haddad, J. C. Lorenz, Y. Xu, H. Wu, L. Zhang, Z. S. Han , F. Roschangar, J. J. Song, N. K. Yee, and C. H. Senanayake, J. Org. Chem., 2019, DOI: 10.1021/acs.joc.8b03040
|
|
|
|
Publication 62: Organic Photocatalysis for the Radical Couplings of Boronic Acid Derivatives in Batch and Flow
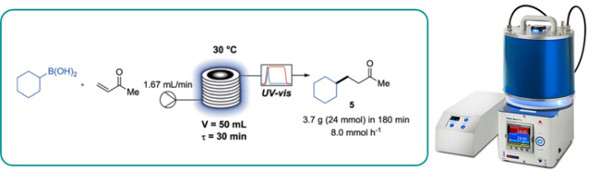
An acridium-based organic photocatalyst is reported to be an efficient replacement for iridium-based photocatalysts to oxidise boronic acid derivatives by a single electron process.
This was exemplified by the synthesis of four active pharmaceutical ingredients (APIs).
A straightforward scale up approach using continuous flow photoreactors is also reported affording gram an hour throughput.
The Uniqsis Flow-UV inline spectrometer was utilised to conveniently monitor steady state.
|
|
|
|
Publication 61: Continuous Flow Synthesis of Indoles by Pd-Mediated Reduction of o-Nitrostilbenes with Carbon Monoxide
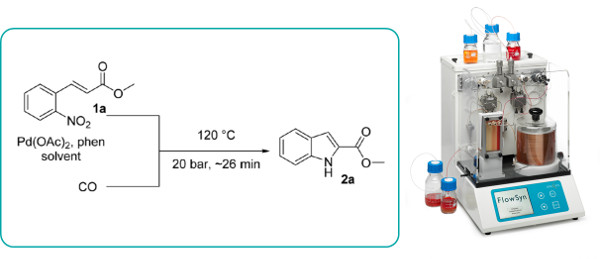
The Pd-mediated reduction of o-nitrostilbenes in the presence of carbon monoxide is reported to proceed regioselectively under pressurised flow-through conditions to cleanly afford indoles.
A variety of substrates are tolerated and the reaction proceeds significantly faster than in batch and produces carbon dioxide as the only stoichiometric side-product.
|
|
|
|
Publication 60: Chemoselective Ru-Catalysed Hydrogen Transfer Oxidation of Secondary Alcohols
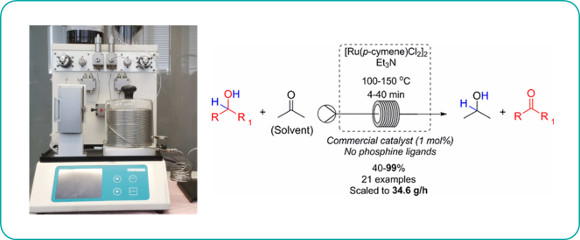
An operationally straightforward continuous flow-through protocol for the chemoselective hydrogen transfer oxidation of secondary alcohols is reported.
The method uses a low loading (1 mol%) of [Ru(p-cymene)Cl2]2in the presence of triethylamine in acetone as both solvent and oxidant and could be scaled up to a throughput of approximately 35g/h.
Soluble residual ruthenium could be effectively removed from the product using QuadraSil-MP.
R. Labes, C. Battilocchio, C. Mateos, G. R. Cumming, O. de Frutos, J. A. Rinco´n, K. Binder, S. V. Ley, Org. Process. Res. Dev., 2017, ASAP
|
|
|
|
Publication 59: 3D Printed Continuous-Flow Column Reactors for Synthesis
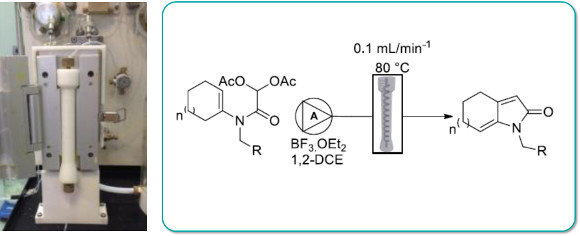
Custom designed polypropylene reactor columns compatible with the Uniqsis FlowSyn were manufactured using low-cost 3D printing techniques.
The reactors could be used several times to perform both SNAr reactions at high temperature in DMF and intramolecular acylal (IAC) cyclisation reactions in the presence of BF3:OEt2 in dichloroethane in good yields.
This work demonstrates the potential to develop and utilise customised low-cost 3D printed reactors in synthetic chemistry.
Z. X. Rao, B. Patel, A. Monaco, Z. J. Cao, M. Barniol-Xicota, E. Pichon, M. Ladlow, S. Hilton, Eur. J. Org. Chem., 2017, 44, 6499
|
|
|
|
Publication 58: Continuous Flow Ring-closing Metathesis
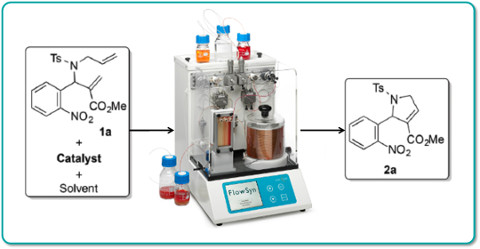
Ring-closing metathesis (RCM) is an important reaction in synthesis.
Lamaty's group in Montpellier have demonstrated that useful dihydro-pyrrole-carboxylates can be prepared by ruthenium-catalysed RCM with a residence time of only 1 min. at 120oC. Such intermediates are useful precursors for the synthesis of drug-like pyrrole and pyrroloquinoline derivatives.
Significantly, the continuous flow-through conditions proved to be general and readily scalable to yield, for example up to 10g of a representative dihydro-pyrrole-carboxylate in 91% yield in only 37 mins.
Moreover, dimethyl carbonate was determined to be an excellent green solvent for this process.
M. Drop, X. Bantreil, K. Grychowska, G. U. Mahoro, E. Colacino, M. Pawlowski, J. Martinez, G. Subra, P. Zajdela, F. Lamaty, Green Chem., 2017, Advance Article DOI: 10.1039/C7GC00235A
|
|
|
|
Publication 57:Silica-Supported Silver Nitrate as a Highly Active Dearomatizing
Spirocyclization Catalyst
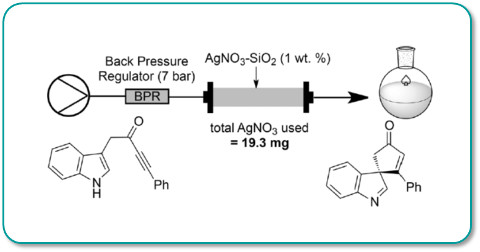
Silver nitrate pre-absorbed onto silica gel has been shown to be an effective and efficient catalyst for promoting rapid and efficient de-aromatisation/spirocyclisation of alkyne tethered heteroaromatics.
Under flow-through conditions >20g of substrate was converted to product in quantitative yield using only 19mg of silver nitrate.
|
|
|
|
Publication 56: Synthesis of a Precursor to Sacubitril Using Enabling Technologies
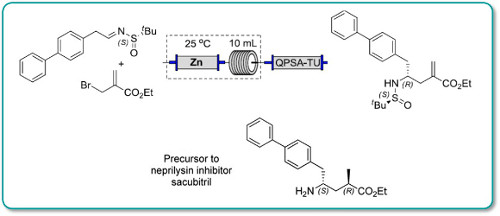
Continuous flow methodology has been used to enhance several steps in the synthesis of a precursor to Sacubitril.
In particular, a key carboethoxyallylation benefited from a reduced processing time and improved reproducibility, the latter attributable to avoiding the use of a slurry as in the batch procedure. Moreover, in batch exothermic formation of the organozinc species resulted in the formation of side products, whereas this could be avoided in flow because heat dissipation from a narrow packed column of zinc was more efficient
|
|
|
|
Publication 55: Multi-step Continuous Flow Pyrazole Synthesis via a Metal-free Amine-redox Process
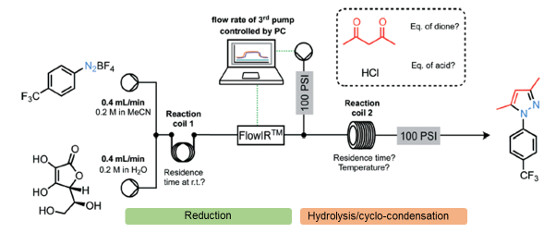
A versatile multi-step continuous flow synthesis for the preparation of substituted pyrazoles is presented.
The automated synthesis utilises a metal-free ascorbic acid mediated reduction of diazonium salts prepared from aniline starting materials followed by hydrolysis of the intermediate hydazide and cyclo-condensation with various 1,3-dicarbonyl equivalents to afford good yields of isolated functionalised pyrazole products.
The synthesis of the COX-2 selective NSAID was demonstrated using this approach.
|
|
|
|
Publication 54: Polymer Conjugations via “Click” Chemistry Employing Microreactor Technology
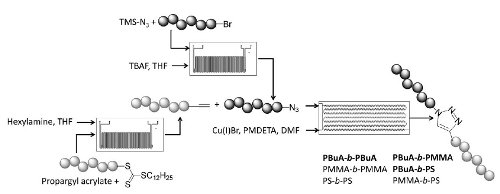
A microreactor system was used to prepare end-cap modified polymers (alkynes and azides) which were then employed in block co-polymerisations using a trazole linking group prepared under copper catalysed 'Click' conditions.
Good coupling efficiencies are observed and notably whereas the corresponding batch process takes several hours to reach completion the flow process is complete in only 40 minutes.
The results indicate that the use of a continuous flow reactor for end group modifications as well as click reactions has clear benefits towards the development and improvement of well-defined polymer materials.
|
|
|
|
Publication 53: Automated Hydrogen Transfer in Flow using a Monolithic Iridium Catalyst
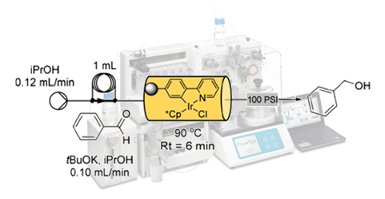
An immobilised monolithic iridium hydrogen transfer catalyst has been developed for use in flow based processing. The monolithic construc thas been used for several redox reductions demonstrating excellent recyclability, good turnover numbersand high chemical stability giving negligible metal leaching over extended periods of use.
A FlowSyn Auto-LF system was employed to automatically process a library of 40 aldehydes and ketones.
|
|
|
|
Publication 52: Flow Epoxidation using an Efficient Mo based PS-AMP Catalyst
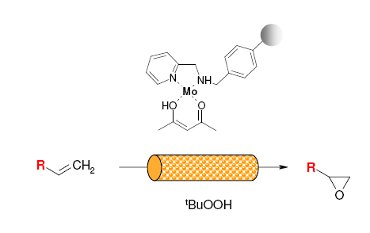
A polystyrene 2-(aminomethyl)pyridine supported molybdenum(VI) complex (Ps·AMP·Mo) has been prepared, characterised and used as a catalyst for epoxidation of 1-hexene and 4-vinyl-1-cyclohexene (4-VCH) using TBHP as an oxidant.
The continuous epoxidation in a FlowSyn reactor has shown considerable time savings, high reproducibility and selectivity along with remarkable improvements in catalyst stability compared with the reactions carried out in a batch reactor.
|
|
|
|
Publication 51: Copper-Catalyzed Formation of C-O Bonds by Direct alpha-C-H Bond Activation of Ethers
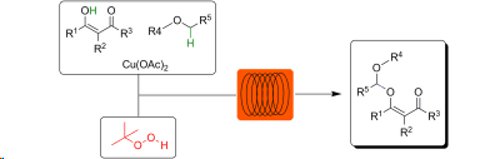
Peroxides and ethers in flow: 2-Carbonyl-substituted phenols and ß-ketoesters react safely with ethers in a microreactor environment using a copper catalyst and an organic peroxide (TBHP).
Closely comparable results were obtained utilising either a continuous flow or microwave reactor.
This protocol results in unsymmetrical acetal scaffolds not easily available otherwise.
|
|
|
|
Publication 50: Continuous Flow Metathesis for Direct Valorization of Cocoa Butter Triglyceride Food Waste
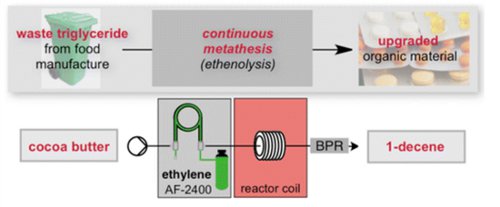
The direct chemical conversion of cocoa butter triglycerides, a material available as a postmanufacture waste stream from the food industry, to 1-decene by way of ethenolysis is reported. The conversion of the raw waste material was made possible by use of 1 mol % of the [RuCl2(iBu-phoban)-2-(3-phenylindenyl)] catalyst.
The process has been investigated in both batch and flow conditions, where the latter approach employs a Teflon AF-2400 tube-in-tube gas–liquid membrane contactor to deliver ethylene to the reaction system.
These preliminary studies culminate in a continuous processing system, which maintained a constant output over a 150 min period tested.
C. Schotten, D. Plaza, S. Manzini, S. P. Nolan, S. V. Ley, D. L. Browne, Alexei Lapkin, ACS Sustainable Chem. Eng., 2015, 3 (7), pp 1453–1459
|
|
|
|
Publication 49: A Continuous Flow Aerobic Anti-Markovnikov Wacker Oxidation
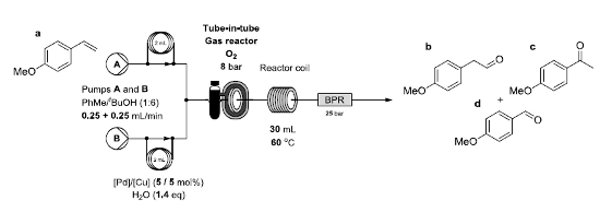
An aerobic anti-Markovnikov Wacker oxidation for the flow-synthesis of arylacetaldehydes is reported.
In the process, flow chemistry techniques have provided a means to control and minimise the over-oxidation of sensitive products.
The reaction showed general applicability to various functionalised styrenes and provided a process capable of a multi-gram scale.
|
|
|
|
Publication 48: Lithium Dicyclohexylamide - Practical and Economic Lithiations of Functionalised Arenes and Heteroarenes in Flow
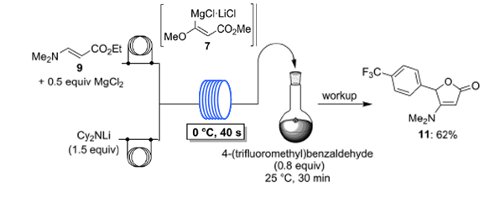
The economic amide base lithium dicyclohexylamide (Cy2NLi) allows fast and convenient (40s, 0°C) in situ trapping flow metalations of a broad range of functionalized arenes, heteroarenes and acrylate derivatives in the presence of various metal salts (ZnCl2·2LiCl, MgCl2, LaCl3·2LiCl).
The resulting Zn-, Mg- or La-organometallic intermediates are trapped with various electrophiles in high yields.
These flow metalations are easily scaled-up without further optimization.
|
|
|
|
Publication 47: Practical Regioselective Flow Metalation and Trapping with Electrophiles using TMPLi
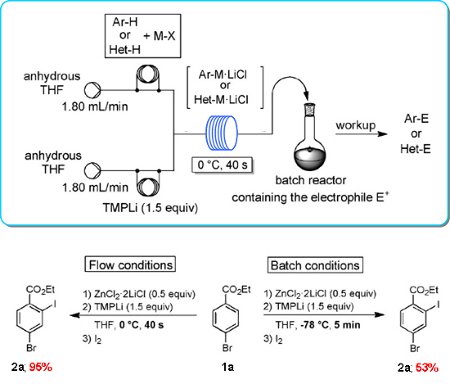
The flow metalation of various arenes and heteroarenes involving an in situ trapping with metal salts (ZnCl2·2LiCl, MgCl2, CuCN·2LiCl, LaCl3·2LiCl) under very convenient conditions (0°C, 40s) is reported. The resulting Mg, Zn, Cu, or La organic species are trapped with various electrophiles in high yields.
In several cases, unusual kinetically controlled regioselectivities are obtained.
All these flow metalations can be scaled up simply by extending the reaction time and without further optimization. The reaction scope of such flow metalations is considerably broader than that of the corresponding batch procedures.
|
|
|
|
Publication 46: Investigating Scale-Up and Further Applications of DABAL-Me3 Promoted Amide Synthesis
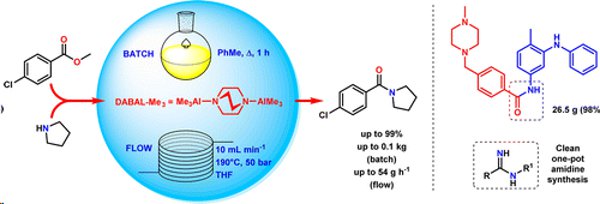
Amides, amidines and amidrazones have been prepared on up to 100g scale from the corresponding esters using DABAL-Me3.A derivative of Imatinib (Gleevec) was prepared on a 26g scale.
Continuous flow methodology was shown to provide a useful method for larger scales (productivities of >50 g h–1) and could be performed successfully on a smaller laboratory scale using a FlowSyn flow synthesiser.
D. S. Lee, Z. Amara, M. Poliakoff, T. Harman, G. Reid, B. Rhodes, S. Brough, T. McInally, S. Woodward, Org. Process Res. Dev., 2015: DOI: 10.1021/acs.oprd.5b00101
|
|
|
|
Publication 45: Substituted Allenes Prepared by Flow-Generated Diazo Compounds
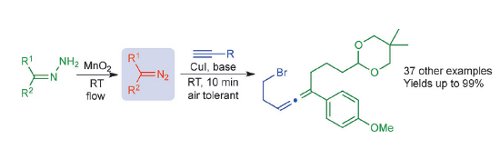
A versatile and mild, room temperature copper-catalyzed coupling reaction between unstabilized diazo compounds and terminal alkynes is reported.
The method provides di- and trisubstituted allenes with high functional-group tolerance.The diazo compounds are generated from hydrazines on demand under flow-through conditions.
|
|
|
|
Publication 44: Synthesis of Bimetallic Nano-Alloys under Continuous Flow Conditions
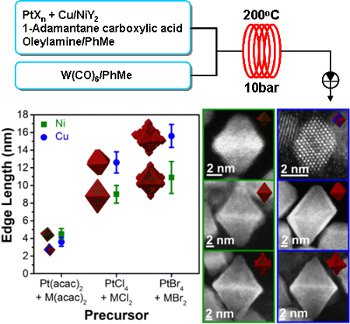
A flow-through strategy for the synthesis of bimetallic nano-alloys under pressurised superheated conditions using a Uniqsis Multi-X flow chemistry system which permits substantial control of key physical properties of the particles is presented.
In particular, it was found that particles could be produced with a tuneable size distribution and shape.
Moreover, ligand dependent control of surface morphology and the distribution of the metals throughout the particles was also possible.In addition, the present strategy was found to be scalable and therefore potentially suitable for the industrial production of well-defined nano-alloys.
|
|
|
|
Publication 43: Developing Multi-kilogram Continuous Flow Cyclopropanation
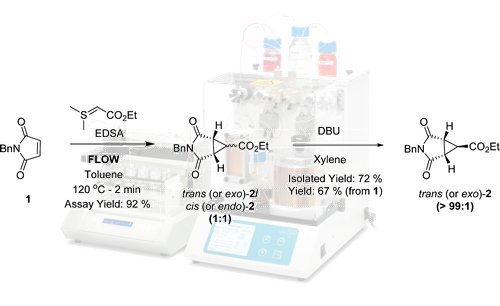
Following reaction optimisation using a FlowSyn, a convenient and high yielding flow cyclopropanation process using EDSA following by a base mediated isomerisation was developed to afford trans-(dioxo)-azabicyclo-[3.1.0]-hexane carboxylate
Efficient controlled mixing was shown to be a key requirement to obtain a high yield and minimise side-product formation.
In this way, the reaction yield was increased significantly under flow conditions in comparison to batch and the robustness and reproducibility of the process was demonstrated by the synthesis of this key intermediate on a multi-kilogram scale.
F. G. Buono, M. C. Eriksson, B.-S. Yang, S. R. Kapadia, H. Lee, J. Brazzillo, J. C. Lorenz, L. Nummy, C. A. Busacca, N. Yee , C. Senanayake, Org. Process Res. Dev., 2014, 18 (11), pp 1527–1534
|
|
|
|
Publication 42: Improved Photo-Induced Cobalt-Mediated Radical Polymerization in Continuous Flow Photoreactors
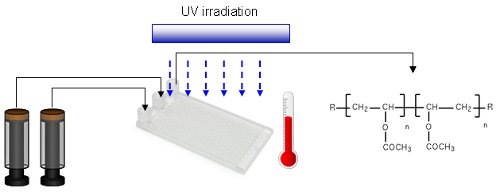
The cobalt-mediated radical polymerisation of vinyl acetate has been shown to be a scalable UV reaction that is significantly improved when carried out under continuous flow-through conditions. Reaction rates are considerably increased and product quality is improved such that extensive cross-linking and the formation of insoluble polymers can be largely avoided.
Co-polymerisation of vinyl acetate with 1-octene was also improved and the flow reactor was found to be particularly useful for investigating the kinetics of these radical polymerisation processes.
Interestingly, when translating from batch to flow, the rate of polymerisation was observed to be approximately 4 fold faster even in the absence of UV irradiation.
|
|
|
|
Publication 41: Selective and Efficient Oxidation of Thioethers to Sulfoxides and Sulphones under Segmented and Continuous Flow-through Conditions
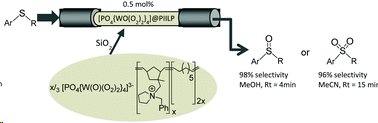
The peroxometalate-based polymer immobilized ionic liquid phase catalyst [PO4{WO(O2)2}4]@PIILP was immobilised on silica gel and shown to be an efficient catalyst for the oxidation of sulfides by hydrogen peroxide to either sulfoxides or sulfones under either continuous or segmented flow-through conditions.
In MeOH 92% selectivity for sulfoxide was achieved at 96% conversion and a Rt=4 min, whilst 96% selectivity for sulfoxide was observed at 96% conversion in acetonitrile for Rt=8 min.
Minimal catalyst leaching was observed permitting 6.5g of substrate to be processed over an 8h period with a stable activity/selectivity profile (TON=46,428). Moreover, a single catalyst cartridge could be used for the consecutive oxidation of multiple substrates giving activity-selectivity profiles that matched those obtained with fresh catalyst.
|
|
|
|
Publication 40: Multistep Flow Synthesis of 5-Amino-2-aryl-2H-[1,2,3]-triazole-4-
carbonitriles
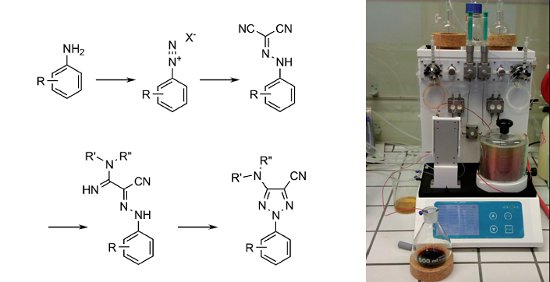
Using the Uniqsis FlowSyn flow chemistry system researchers from the UCB Biopharma. Belgium have developed a flow synthesis of 2-substituted 1,2,3-triazoles that demonstrates improvements over the conventional batch route.
The route involves the diazotisation of anilines and condensation with malononitrile followed by the nucleophilic addition of ammonia or an alkylamine and finally a novel copper catalysed cyclisation. The intermediate azide was generated and consumed in situ which enabled safe scale up under the flow-through conditions employed.
|
|
|
|
Publication 39: A Continuous Flow Chemistry Platform for Low Temperature Reactions
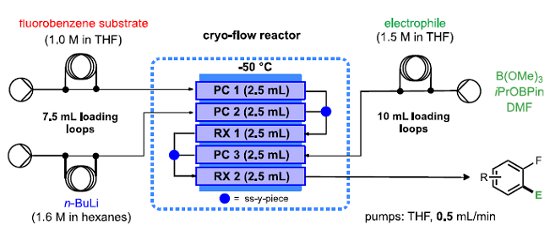
A low temperature reactor platform that facilitates metalation followed by electrophilic quenching, and related reactions under continuous flow-through conditions has been developed.
Pre-cooling loops are incorporated for all 3 reactor input streams and gaseous reagent inputs can be easily introduced by attaching a 'tube-in'tube' presaturation module.
All of the coil reactors and flow chemistry equipment described are are available from Uniqsis Ltd
|
|
|
|
Publication 38: Continuous Flow alpha-Trifluoromethylation of Ketones under Metal-Free Visible Light Photoredox Catalysis
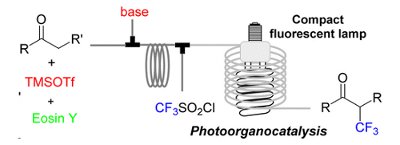
A continuous-flow, two-step procedure for the preparation of a-CF3-substituted carbonyl compounds has been developed using the Uniqsis Binary Pump Module in combination with a lab built photoreactor.
The carbonyl substrates were converted in situ into the corresponding silyl enol ethers, mixed with the CF3 radical source, and then irradiated with visible light using a flow reactor based on transparent tubing and a household compact fluorescent lamp.
The continuous protocol uses Eosin Y as an inexpensive photoredox catalyst and requires only 20 min to complete the two reaction steps.
|
|
|
|
Publication 37: Continuous Flow Magnesiation of Functionalised Heterocycles & Acrylates with TMPMgCl.LiCl
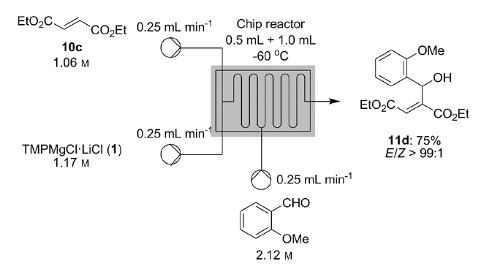
Knochel's group in Munich have recently disclosed how a variety of functionalised heterocycles and sensitive acrylates can be rapidly magnesiated and subsequently quenched with an electrophile under continuous flow-through;conditions using a Uniqsis static mixer/reactor chip.
A key advantage is that, in contrast to typical batch procedures, these reactions required non-cryogenic conditions (typically 25C); moreover the procedure could be quickly scaled to 45 mmol without modification of the reaction conditions.
Metalations under flow-through conditions permited magnesiations that did not afford the desired product under batch conditions and acylates could be magnesiated and quenched to afford products with high stereoselectivities without concomitant polymerisation.
|
|
|
|
Publication 36: Continuous Bromination of Methanesulfones and Methansulfonates using KOBr
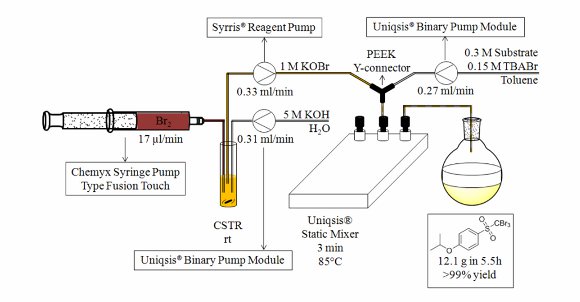
Halomethylsulfonyls constitute a group of commercially important agents with biocidal, herbicidal, antifungal and polymerisation initiating properties that are manufactured industrially on a large scale.
Stevens' group in Belgium have developed a fast and efficient continuous flow-through bromination procedure that utilises KOBr as the brominating agent that is formed on demand.
This process is expedited by emulsion formation of the biphasic reaction mixture in a Uniqsis glass static mixer chip/reactor to afford quantitative yields of halogenated methylsulfones and methane sulfonates in through-puts of up to 53g per day using a laboratory scale system as a proof of concept.
|
|
|
|
Publication 35: Continuous Flow Synthesis of alpha-Halo Ketones: Building Blocks for Anti-retroviral Agents
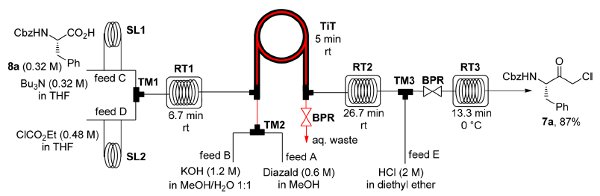
Chiral alpha-halo ketones derived from N-protected amino acids are key building blocks for the synthesis of HIV protease inhibitors such as atazanavir used in HAART combination therapy.
Kappe and De Souza have reported a continuous flow through route to these intermediates which utilises a tube-in-tube reactor to introduce diazomethane generated on demand into the reaction stream containing mixed anhydride derivatives of N-protected amino acids. The resulting alpha-diazo ketones are then decomposed with HCl or HBr to afford the corresponding alpha-halo ketones.
This process allows the safe generation, separation and use of diazomethane in a continuous integrated multi-step synthesis of important API intermediates.
|
|
|
|
Publication 34: Synthesis and Scale-up of Boronic Acids using a Flexible Flow-Through Chemistry Platform
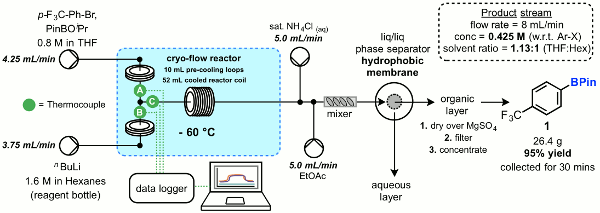
Boronic acids are important and widely utilised synthetic intermediates. However, often the one that you really want isn't commercially available and you have to make it - on scale. Continuous processing provides an attractive option whereby precise temperature control can afford high purity products.
A series of aryl boronic acids have been prepared at a rate of production of up to 50 g/h by continuous lithium-halogen exchange followed by in situ borylation.
Larger internal diameter coil reactors were found to be necessary to prevent blockages and a flexible flow reactor platform employing an easily exchanged range of pre-cooling and reactor coils in conjunction with a Polar Bear cryo-reactor was utilised and demonstrated to provide effect control of reaction temperatures. The use on an integrated in-line continuous liquid-liquid separation device was also exemplified to effect continuous purification of the product stream.
|
|
|
|
Publication 33: Automated Flow-Through Synthesis of Photovoltaic Quality Colloidal PbS Quantum Dots
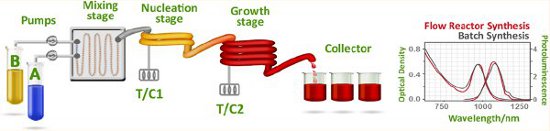
Superior high quality PbS nanocrystals were obtained using a dual-temperature-stage nucleation/growth flow continuous flow-through process that exhibited high photoluminescence quantum yield (50%) and narrow full width-half-maximum.
The PbS CQDs could be used to fabricate solar cells having performance similar to that of comparable batch-synthesized nanoparticles.
The flow reactor approach, with its versatility and ability to rapidly screen multiple parameters, offers an attractive path to the automated and scalable synthesis of CQDs for photovoltaics and, more broadly, active optoelectronics.
J. Pan, A. O. El-Ballouli, L. Rollny, O. Voznyy, V. M. Burlakov, A. Goriely, E. H. Sargent, O. M. Bakr, ACS Nano, 2013, DOI: 10.1021/nn404397d
|
|
|
|
Publication 32: Continuous Flow Generation and Reactions of Anhydrous Diazomethane Using a Teflon AF2400 'Tube-in-Tube' Reactor

A continuous process for generation, separation and reaction of anhydrous diazomethane using an AF2400 'tube-in-tube' reactor has been developed.
This technique allows safe and scalable reactions with dry diazomethane to be performed on a laboratory scale and is exemplified by the preparation of methyl esters, aziridines, pyrazolines and alpha diazoketones, and by the N-methylation of heterocycles.
|
|
|
|
Publication 31: Rapid Continuous Flow Synthesis of Silver Nanocubes and Nanospheres
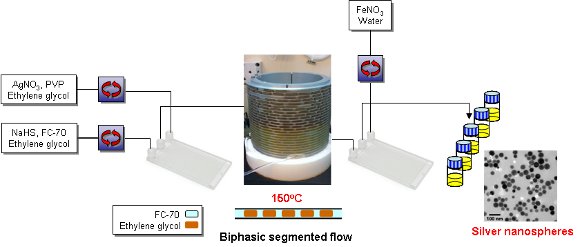
A scalable continuous process for the synthesis of high-quality plasmonic single crystal nanocubes (AgNCs) and nanospheres (AgNSs) under biphasic segmented flow-through conditions has been developed.
Nanocubes could be produced with a controllable edge length in the range 20 to 48 nm by varying the synthesis conditions.
The nanospheres were produced by continuous in-line etching of the nanocubes using ferric nitrate.
|
|
|
|
Publication 30: Continuous-Flow Transfer Hydrogenation of Olefins using in situ Generated Diimide
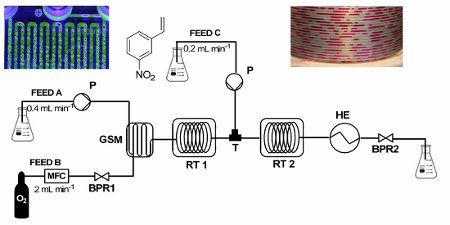
No catalyst required! A highly efficient, catalyst-free process for the selective reduction of alkenes has been developed.
The process uses a gas–liquid segmented flow regime to generate diimide in situ from hydrazine monohydrate and molecular oxygen under safe operating conditions and dramatically enhanced this atom-economical reaction, resulting in short processing times.
|
|
|
|
Publication 29: Scaled-up Continuous Flow Synthesis with Gases - Fanetizole Synthesis with Ammonia
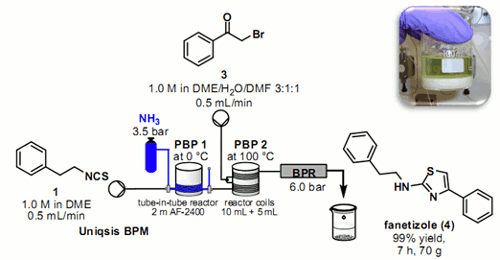
Researchers at Cambridge have shown how it is possible to calibrate a 'tube-in-tube' reactor containing ammonia gas using a simple in-line colourimetric titration technique.
This information was then used to deliver an ammonia solution of stoichiometrically to effect the telescoped 2 stage synthesis of the anti-inflammatory agent Fanetizole.
The automated continuous flow synthesiser was able to produce drug substance at a rate of approximately 10 g per hour, isolating the product by direct precipitation from the outflow reaction stream.
|
|
|
|
Publication 28: Flow Syntheses of Styrenes, Unsymmetrical Stilbenes & Branched Aldehydes

A palladium mediated flow-through synthesis of styrenes which consumed ethylene delivered via a 'tube-in-tube' reactor was developed.
This styrene could then be utilised in a second Heck cross-coupling to afford unsymmetrical stilbenes.
Alternatively, following in-line removal of an inhibitory contaminant the styrene synthesis could be telescope with a rhodium mediated hydroformylation using syngas introduced through another 'tube-in-tube' reactor to afford branched aldehyde products directly from aryl halides in a single process.
S.L. Bourne, M. O’Brien, S. Kasinathan, P. Koos, P. Tolstoy, D.X. Hu, R.W. Bates, B. Martin, B. Schenkel, S.V. Ley, ChemCatChem., 2013, 5, 159-172.
|
|
|
|
Publication 27: Flow Syntheses of GABAA Agonists and their Biological Evaluation through the use of In-line Frontal Affinity Chromatography
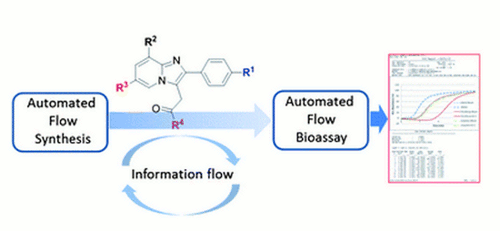
The flow of information between chemical and biological research can present a bottleneck in pharmaceutical research.
We report here on the use of flow processes to perform synthesis and biological evaluation in an integrated manner. As proof of concept, a flow synthesis of a series of imidazo[1,2]-pyridines, including zolpidem and alpidem, was developed using the FlowSyn Auto-LF combinatorial library synthesis platform and connected to a Frontal Affinity Chromatography screening assay to investigate their interaction with Human Serum Albumin (HSA).
|
|
|
|
Publication 26: Flow Synthesis of the Neurotensin-1 Receptor Probe Meclinertant (SR48692)
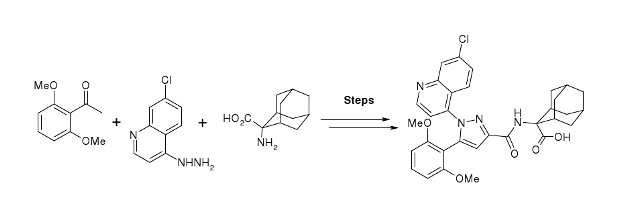
A synthesis of the neurotensin 1 receptor probe Merclinertant (SR48692) has been reported using a range of continuous flow through synthesis, in-line reaction monioring and purification techniques. This strategy has been contrasted with a more conventional batch synthesis approach.
Notably the safe use of phosgene gas (generated in situ), the superheating of solvents to accelerate reaction rates, the processing of a reagent suspension under continuous flow-through conditions and the application of semi-permeable membrane technology to facilitate work-up and purification were all techniques that could be beneficially applied in the synthetic scheme.
|
|
|
|
Publication 25: 2-Amino-Adamantane-2-Carboxylic Acid

A new flow-through, multi-step synthesis of the achiral unnatural amino acid 2-amino-2-adamantane-2-carboxylic acid has recently been reported by Ley's group.
The synthetic route employs a series of transformations that employ different mesoflow reactors. Notably all intermediates were isolated in high purity without the need for column chromatographic purification, thereby rendering the route suitable for scale-up.The automated flow synthesiser could be used successfully under the harsh reaction conditions.
|
|
|
|
Publication 24: Direct Synthesis of Amides under Flow-Through Conditions
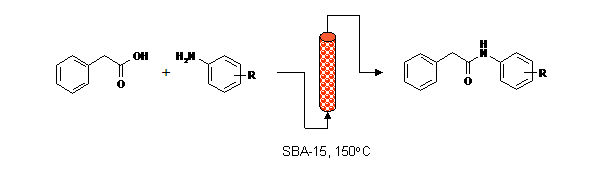
Amides can be synthesised continuously by condensation of carboxylic acids and amines under flow-through conditions in the presence of activated catalysts prepared from structured mesoporous silicas.
Structured mesoporous silicas (SBAs) are benign materials that were found to offer increased reaction rates in comparison to chromatographic silicas and are therefore particularly suited to continuous processing.
|
|
|
|
Publication 23: Stereospecific synthesis of Alpha-Hydroxyacids by Diazotisation of Amino Acids

Alpha-hydroxy acids are useful chiral starting materials in total synthesis. They may be conveniently obtained by direct diazotisation and hydrolysis of the corresponding alpha-amino acids.
Under continuous flow-through conditions, diazotisation may be achieved safely on scale.
In addition, a flexible and fully automated protocol for continuous liquid-liquid extraction is presented which allows for the continuous extractive work-up and isolation of multi-gram quantities of these useful compounds.
|
|
|
|
Publication 22: Efficient Reduction of Nitroarenes under Continuous Flow Conditions using an In-situ generated Nanoparticular Iron Oxide
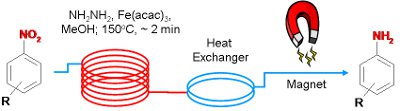
An efficient continuous flow-through process for the reduction of nitroarenes in the presence of nanoparticular Fe2O3 is reported.
The nanoparticles were conveniently produced in-situ using soluble Fe(acac)3 in the presence of hydrazine as the reducing agent and remain in solution throughout. After product isolation, aggregation of the nanoparticles occurs facilitating their removal using a magnet.
Notably multi-gram quantities of compounds could be readily processed.
|
|
|
|
Publication 21: Continuous Flow Reaction Monitoring using a Miniature On-line Mass Spectrometer
A revolutionary new miniature ESI mass spectrometer has been used to directly monitor on-line the products emerging from a mesoscale flow synthesiser.
In this way, it was possible to observe unstable reaction intermediates and by monitoring trends in selected ion peak heights it was possible to quickly identify optimal reaction conditions for synthesis of the desired reaction product.
In general, this approach provides a detailed and informative snapshot of a flow-through chemical process. Analysis by on-line MS is usefully complementary to alternative methods of both on-line and in-line monitoring of flow chemistries.
D. L. Browne, S. Wright, B. J. Deadman, S. Dunnage, I. R. Baxendale, R. M. Turner, S. V. Ley, Rapid Commun. Mass Spectrom., 2012, 26, 1999.
|
|
|
|
Publication 20: SynGas-Mediated Hydroformylation of Styrenes under Flow-Through Conditions
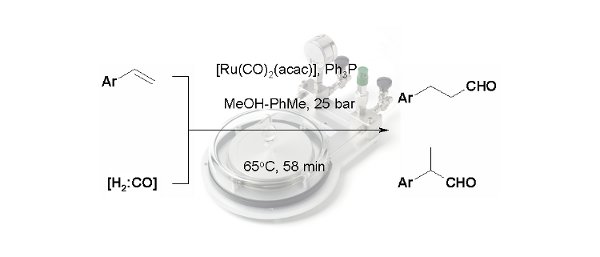
A continuous flow-through approach to the hydroformylation of alkenes using SynGas (1:1 H2:CO) under homogeneous conditions in a 'tube-in-tube' reactor under conditions is reported.
A range of conditions were studied for a model substrate to determine the dependence on reaction temperature, residence time, gas pressure, solvent and rhodium pre-catalyst/ligand composition. In this way, it was possible to identify conditions that afforded the desired aldehyde product in both high yield and with high selectivity.
Moreover the approach was extended to demonstrate who the starting styrene could also be formed under continuous flow-through conditions prior to hydroformylation.
S. Kasinathan, S. L. Bourne, P. Tolstoy, P. Koos. M. O'Brien, R. W. Bates, I. R. Baxendale, S. V. Ley, SynLett, 2011, 18, 2648.
|
|
|
|
Publication 19: Small Molecule Library Synthesis using Segmented Flow
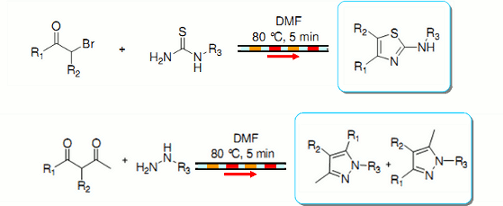
A straightforward strategy for the rapid synthesis of compound libraries thiazoles and pyrazoles under continuous flow-through conditions is presented.
Sequential reactions were performed in small segments ('plugs') of DMF as reaction solvent, each segment being separated from its neighbour by a plug of immiscible fluorous solvent (F-40).
To avoid cross-contamination, each reaction segment was followed by a segment of pure DMF solvent.
Similar results were obtained compared to those using a batch microwave, but the individual reactions were able to be performed more rapidly in a sequential manner.
|
|
|
|
Publication 18: Continuous Production of Oxamic Acid Derivatives in the Mild, Metal-free reduction of Diazonium Salts to Hydrazines
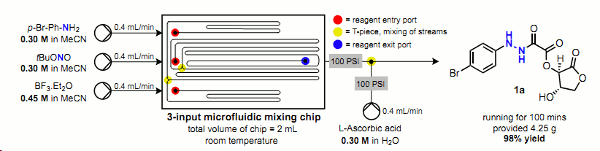
The use of ascorbic acid for the mild, metal-free reduction of in situ formed diazonium salts is presented.
The oxamic acid derivatives thus formed have the potential to act as latent hydrazines.
The ability to perform this chemistry under continuous flow-through conditions reduces considerably the risk of explosion when dealing with both diazonium salts and hydrazines on scale.
|
|
|
|
Publication 17: Synthesis of Mono-acyl 1,4-Diamines from Hydrazoic Acid under Continuous Flow-through Conditions
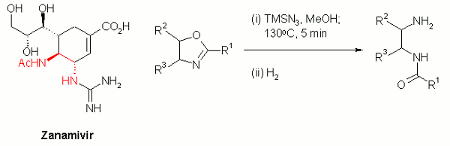
A fast and efficient high temperature protocol for the ring opening of 2-oxazolines to generate mono-acyl 1,4-diamines under continuous flow-through conditions has been reported by Kappe's group in Graz.
Their approach involves the in situ generation of hydrazoic acid by methanolysis of trimethylsilylazide and concomitant reaction with an appropriate 2-oxazoline.
Ring opening of 2-oxazolines under acidic conditions is an important route to the mono-acyl 1,4-diamine moiety found in neuraminidase inhibitors such as GSK's Zanamivir. The ability to perform this reaction in a continuous manner without the potential of accumulating explosive hydrazoic acid in the head space of a batch reactor affords an inherently safer process for large scale synthesis.
(i) B. Gutmann, D. Obermayer, J-P. Roduit, D. M. Roberge, C. O. Kappe, Journal of Flow Chemistry, 2012, (2), 8. (ii) B. Gutmann, J.-P. Roduit, D. Roberge, C. O. Kappe, Chem. Eur. J., 2011, (17), 13146.
|
|
|
|
Publication 16: Preparation of Cisplatin under Microwave and Continuous Flow-through Conditions

Inorganic Flow Chemistry: The preparation of cisplatin, one of the most widely used anti-cancer drugs in the world, is reported on a small scale in a batch microwave reactor and this procedure is scaled up by translation to flow.
Notably, this approach produces cisplatin in high isolated yield free from Magnus' salt, a commonly encountered impurity that is particularly difficult to remove.
|
|
|
|
Publication 15: A Scaleable Continuous Flow-Through Synthesis of Nabumetone and Related 4-Aryl-2-butanones
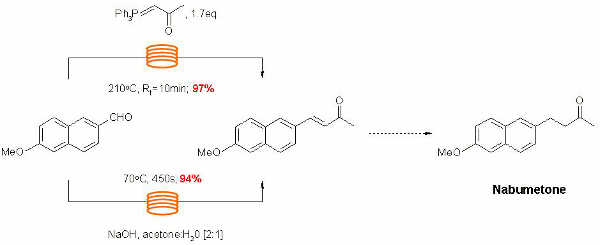
Kappe's group have explored a variety of continuous flow-through approaches to the synthesis of the NSAID Nabumetone and related fragrant 4-aryl-2-butanones.
Their strategy illustrates how reaction optimisation may initially be performed on a small scale in the batch microwave and then directly scaled by translation to electrically heated continuous flow-through devices.
Seemless scaling of the flow synthesis platform up to a throughput of 2.7L/h is also demonstrated as an elegant illustration of the ease and reproducibility that it is possible to achieve through the scaling of continuous flow-though processes without the need for reoptimisation of reaction conditions.
|
|
|
|
Publication 14: Continuous Flow Oxidation of Alcohols with Bleach in the Presence of Catalytic Tetrabutyammonium Bromide

Oxidation: The continuous flow-through oxidation of alcohols to aldehydes, and aldehydes in the presence of methanol to methyl esters is reported to occur in ethyl acetate in the presence of 12.5% bleach and catalytic tetrabutylammonium bromide.
The oxidation conveniently occurs at room temperature within 5-30 min under segmented flow conditions.
|
|
|
|
Publication 13: Synthesis of Aryl Boronic Acids via Low Temperature Metal-Halogen Exchange
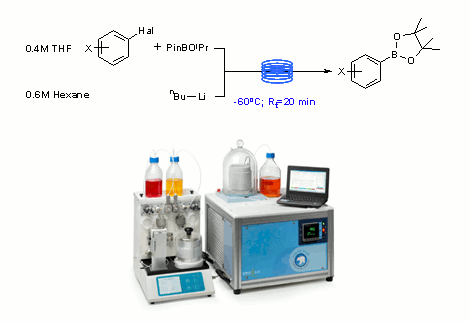
Boronic acids are versatile intermediates in synthesis that are typically used to form C-C, C-O and C-N bonds under metal catalysed conditions.
Although many are commercially available, the formation of boronic acids from bespoke halogenated intermediates is often required, particularly in drug discovery programmes.
Typically this is done via low temperature metal halogen exchange reactions.
In a recent publication, workers from Cambridge demonstrate the use of the new 'Polar Bear' cryo-flow reactor in combination with the direct pumping of n-butyllithium solutions from the reagent bottle for extended periods of time to afford boronic acids on demand in synthetically useful quantities.
|
|
|
|
Publication 12: Palladium Catalysed Alkoxycarbonylation in Flow
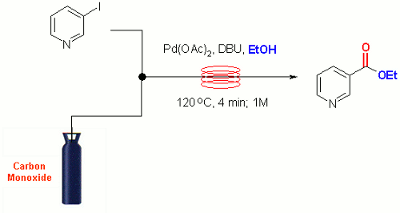
Carbonylation under flow-through conditions: The direct alkoxycarbonylation of aryl halides in the presence of carbon monoxide is an important and useful reaction in synthesis that is well suited to continuous flow-through implementation. Leadbeater’s Group have reported that efficient gas/liquid mixing can be achieved under segmented flow conditions affording high conversion to carbonylated product with a preparatively useful throughput of up to 0.2 mole/hr.
Under the reported conditions reactions are typically complete in 5-10 min at 120oC in the presence of only 0.5 mol% of palladium acetate catalyst.
|
|
|
|
Publication 11: Hydrogenation in flow: Homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas–liquid contact at elevated pressure
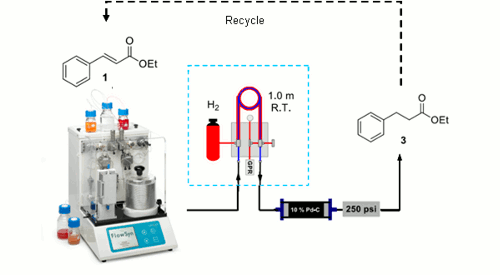
Gas addition under flow-through conditions: Ley’s group in Cambridge, describe a tube-in-tube gas liquid reactor/saturation module based on a gas-permeable Teflon AF-2400 membrane, which allows both heterogeneous and homogeneous catalytic hydrogenation reactions to be efficiently carried out at elevated pressure in flow. The study demonstrates that a continuous hydrogen gas saturated solvent stream can be produced in seconds and that, if necessary, excess gas can be conveniently removed by using a second module to which a partial vacuum is applied.
The introduction of other gases such as CO, CO2, ethene, and ethyne under flow-through conditions can be achieved in a similar way.
|
|
|
|
Publication 10: Safe and Efficient Tetrazole Synthesis in Continuous Flow
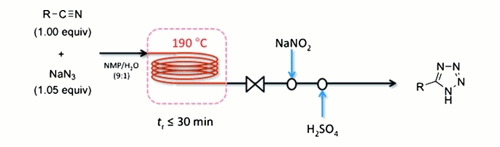
Safer flow: Jamison's group at MIT have reported an expedient synthesis of 5-substituted tetrazoles in flow that is safe, scalable, requires no metal promoter, and uses a near-equimolar amount of sodium azide; yet nonetheless displays a broad substrate scope.
The hazards associated with hydrazoic acid are essentially eliminated, shock-sensitive metal azides such as zinc (II) azide are avoided, and any residual sodium azide is quenched in-line with sodium nitrite.
|
|
|
|
Publication 9: MW and Flow Syntheses of Pseudomonas Quinolone Signal (PQS)
.jpg)
Spring and collaborators at Cambridge have recently reported expedient syntheses of Pseudomonas quinolone quorum sensing signal and related structural analogues using microwave and flow-through protocols.
The flow-through approach facilitated the scale up synthesis of PQS in multi-gram quantities.
|
|
|
|
Publication 8: Bohlmann-Rahtz Cyclodehydration Reaction
.jpg)
In a study to exemplify the use of continuous flow processing to scale up microwave batch reactions, Bagley's group in Cardiff examined the Bohlmann-Rahtz pyridine synthesis. They found that both glass microreactors and stainless steel coil reactors were equally effective, the latter giving a higher throughput.
Discreprancies observed between optimised conditions in batch and flow-through microwave reactors and conductively heated continuous flow reactors were attributed to a lack of certainty in accurately measuring the temperature at the point of reaction in the microwave devices.
|
|
|
|
Publication 7: Preventing Premature Product Precipitation in Flow
The introduction of a suitable organic solvent into the outflow line of a continuous flow reactor is shown to be a useful and pragmatic solution to prevent reaction products from precipitating prematurely.
In particular, this approach facilitates the direct scale up of many microwave protocols without the need to first identify a 'better' solvent or perform extensive reaction optimisation or modification.
|
|
|
|
Publication 6: Permanganate Oxidation in Continuous Flow
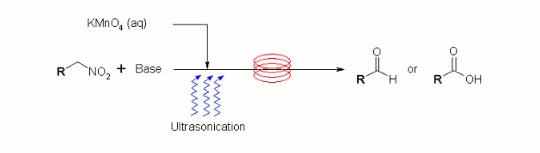
The continuous flow-through oxidation of alcohols and aldehydes in the presence of aqueous potassium permanganate affords carboxylic acids. Under similar conditions nitroalkanes afford the corresponding carbonyls and carboxylic acids.
This process is readily scalable and, when run under pulsed ultrasonication conditions, blockage of the flowpath by the co-formation of manganese dioxide slurries did not occur.
|
|
|
|
Publication 5: Bioinspired Silicas in Flow

Continuous processing offers considerable scope for the preparation of new materials possessing specifically engineered properties.
Patwardhan and Perry have shown how engineered silica particles can be reproducibly prepared under bioinspired conditions in a continuous flow coil reactor. Functionalised silicas prepared encapsulating the enzyme invertase were shown to retain enzymic activity, and additionally silica and colloidal gold composites were prepared that retained optical activity.
Such materials hold considerable potential for application in biocatalysis and photonics.
|
|
|
|
Publication 4: Tetrazole formation
.jpg)
Kappe's group have recently described an elegant method for the continuous flow synthesis of 5-substituted 1H-tetrazole derivatives via addition of in situ generated hydrazoic acid to organic nitriles. Under optimized conditions tetrazole compounds are formed with quantitative conversion in residence times of a few minutes, providing excellent purities and yields of isolated product.
|
|
|
|
Publication 3: Hofman rearrangement
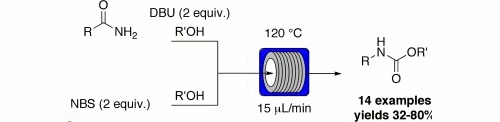
The Hofman rearrangement of amides into their corresponding carbamates works well under flow-through conditions.
|
|
|
|
Publication 2: Alpha-ketoesters
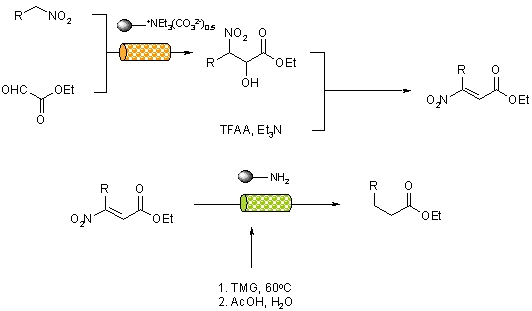
A continuous flow-through approach to alpha-keto esters has been reported that utilises a combination of supported reagents to deliver products in high yield and purity with out the need for further purification.
|
|
|
|
Publication 1: BDA Tartrates
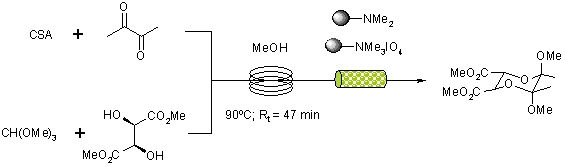
A readily scalable synthesis of BDA protected tartrates has been reported by a continuous flow-through strategy utilising immobilised reagents and scavengers packed into reactor columns.
|